Real-world Evidence: Powerful Analysis Driving Global Trends in Health, Pharmaceuticals and Life Sciences
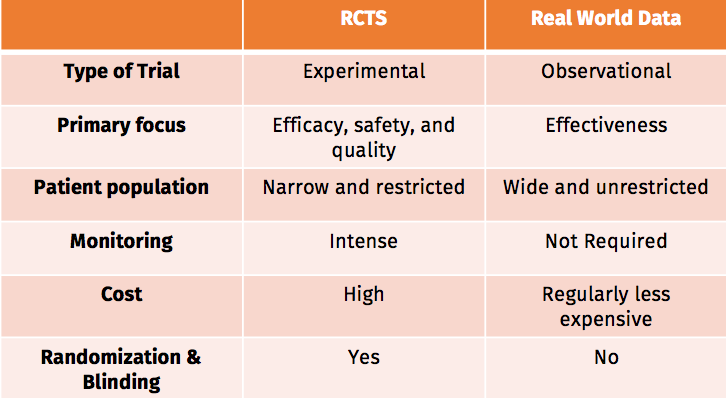
When imagining insights involved with health care and pharmaceuticals, your thoughts might go to scientific data gathered in an experimental setting such as a randomized controlled trial (RCTs). These RCTs are, of course, the gold standard of clinical tests.
However much like it sounds, “real-world data” (RWD) refers to working with real-world observational data, in the wild, and using it to find hidden value in the form of “real-world evidence” (RWE).
Documentation of real-world activities can exist in many forms, including electronic medical records, personal information repositories, claims, and billing activities. The idea of combing through all these sources can be daunting. But there are data mining tools now available.
With the right tools, information and knowledge can be extracted from those sources. Sophisticated language analysis of unstructured elements can normalize this data and allow for a structured analysis of even free-form text.
Konplik (konplik.health) is a semantic platform to analyze RWD in life sciences, pharmaceuticals, and general health care. This data can exist at any touchpoint (email, call center, surveys, social media, etc.) or in any domain-specific repository (internal documents, FDA reports, regulatory data, RFPs, patents…). By specializing in health-related vocabulary, Konplik is uniquely positioned to provide powerful insights into the health care industry.
Supplementing RCTs
RCTs focus on efficacy, safety, and quality. Observational studies using RWD can provide a broader context.
RWE can easily supplement RCTs by informing healthcare policymakers with RWE when formulating treatment pathways and encouraging resource allocation. Extensive RCTs can cost huge amounts of money. Collecting RW data demands a much lower level of funding
True Insight Requires Transformation of Raw Data to Evidence
According to the ISPOR (International Society for Pharmacoeconomics and Outcomes Research) Real World Data is: “data used for decision making that are not collected in conventional randomized controlled trials.”
The terms “real-world data” and “real-world evidence” are too often used interchangeably. However, they are not exact synonym. “data” is a raw material, whereas “evidence” implies that the organization will use discovered information to decide. Data and evidence are the analytical processes that allow us to convert data into evidence and insights and make informed decisions
See a definition of Real-World Evidence in a previous post at Konplik.health.

Benefits of RWD for Health, Pharmaceutical, and Life Sciences Organizations
Investing in an analysis of RWD can have many benefits for the health industry. RWD is the resource to actively listen to the authentic voice of patients and to facilitate collaboration between the healthcare industry and the public sector. In practice, the funding of RWD projects comes primarily from companies. The availability of government funding is significantly lower.
That said, the cost of RWD studies is insignificant as compared to the value. Adding RWD studies allow organizations to consider more contextualized parameters, which may better reveal overall ‘effectiveness’. The insights are robust differentiation in a competitive market.
Decision-makers in the health sector look for more RWE to evaluate the results to base their decisions on.
To accept the distribution of a drug, the purchasing managers require evidence of cost-effectiveness, which is often accompanied by proof of organizational, social, and ethical implications of the product’s contribution.
Most patients are not suitable for randomized clinical trials because of their age or because of their age or because they have comorbidities. As a result, records will not show the information derived from their attention.
Thanks to RWD, on the other hand, we have access to clinical results about a diverse population that reveals the range and distribution of patients observed in clinical practice.
Generalized tools have limitations to accomplish these types of analyses. It is Konplik’s laser focus on the needs of pharmaceuticals, life sciences, and health care that allows for cost-effective and efficient access to insights. Specific domain experience is critical in handling sensitive data and speed to insight.
How can health companies exploit their unstructured data
With a platform such as Konplik, it is possible to identify medical treatments’ costs, efficiency, references to drugs, side effects, or long-term results
To scale the collection and analysis of this RWD, a major challenge must be addressed. The sources of RWD often include text that is not easily understood by machines and algorithms. Properly analyzed, these powerful resources can help improve treatment efficiency (cost, benefits, and risks), detect medical treatment costs, references to drugs, side effects, or long-term results.
With the amount of these records that are presented as textual data, text analytics software is an essential component. Effective text analytics is powering a worldwide trend in Health and Life Sciences to leverage RWD for meaningful discovery and RWE.


Recent Comments